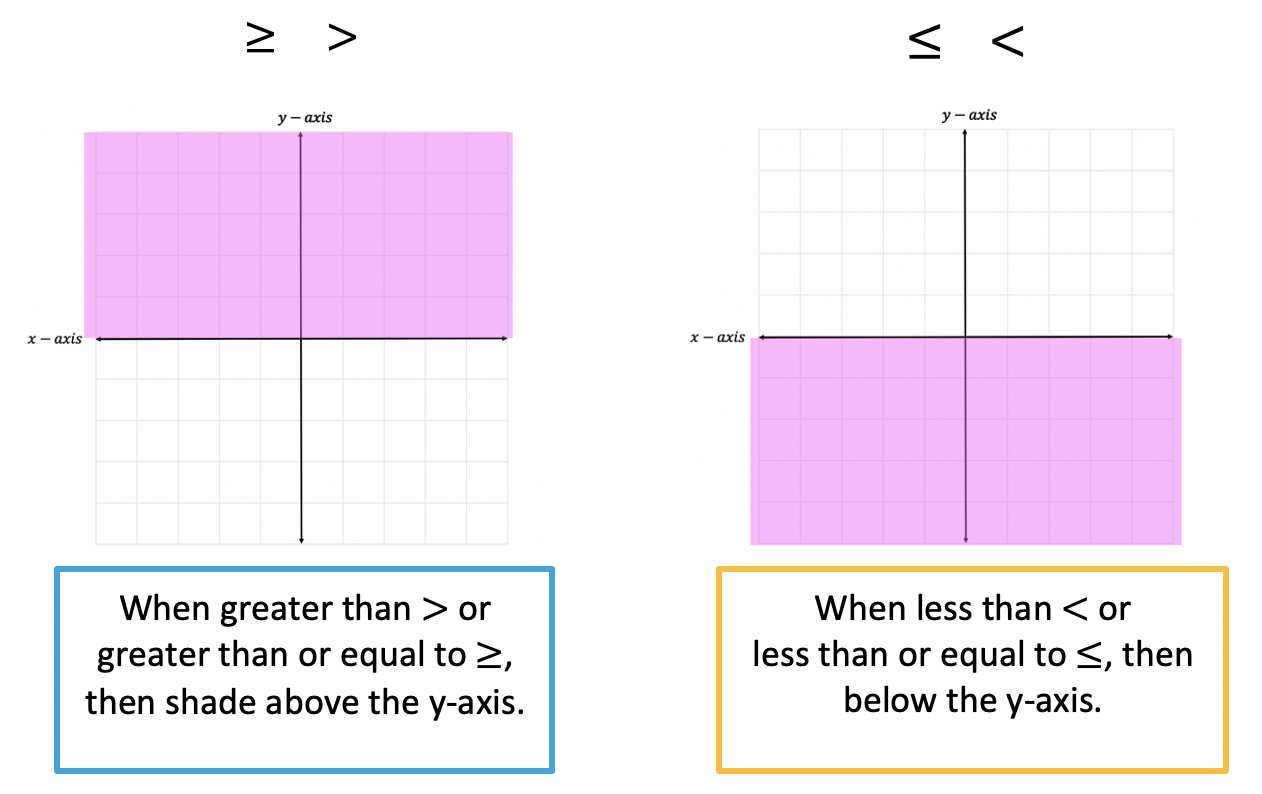
Fun Info About How Do You Know If A Graph Is Weak Or Strong Line Of Best Fit Ti 84 Plus Ce

If the titration is a strong acid with a strong base, the ph at the equivalence point is equal to 7.
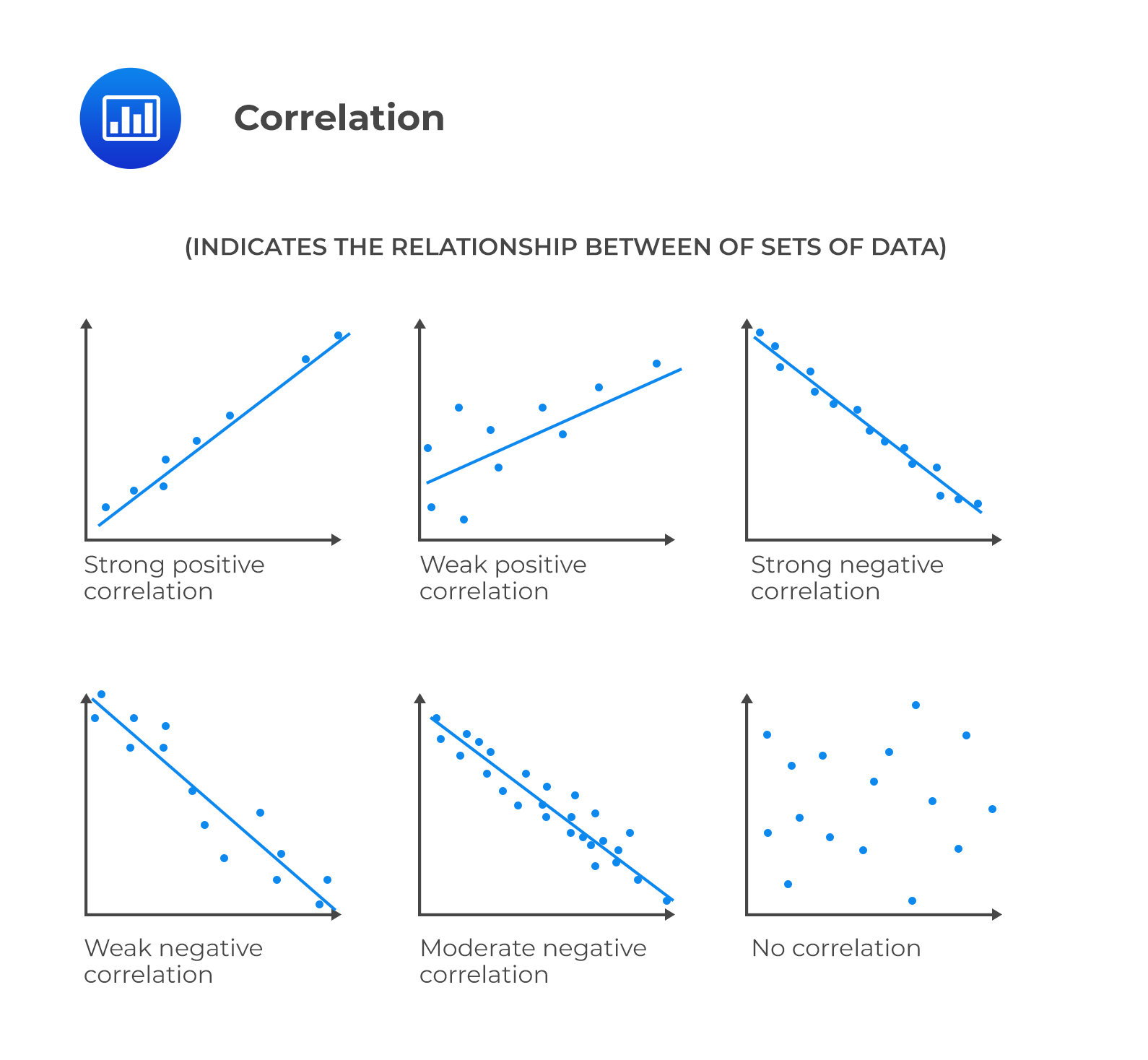
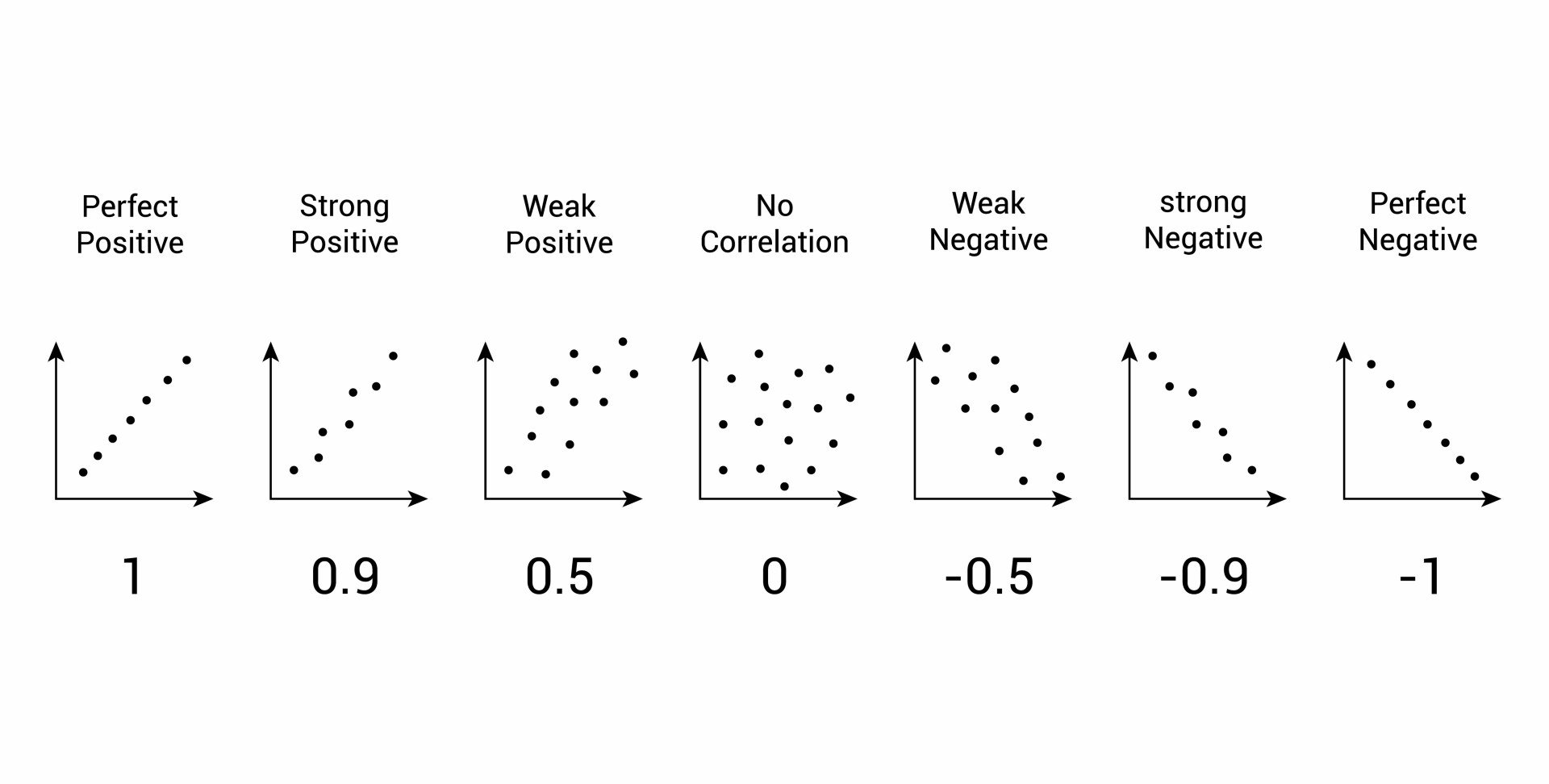
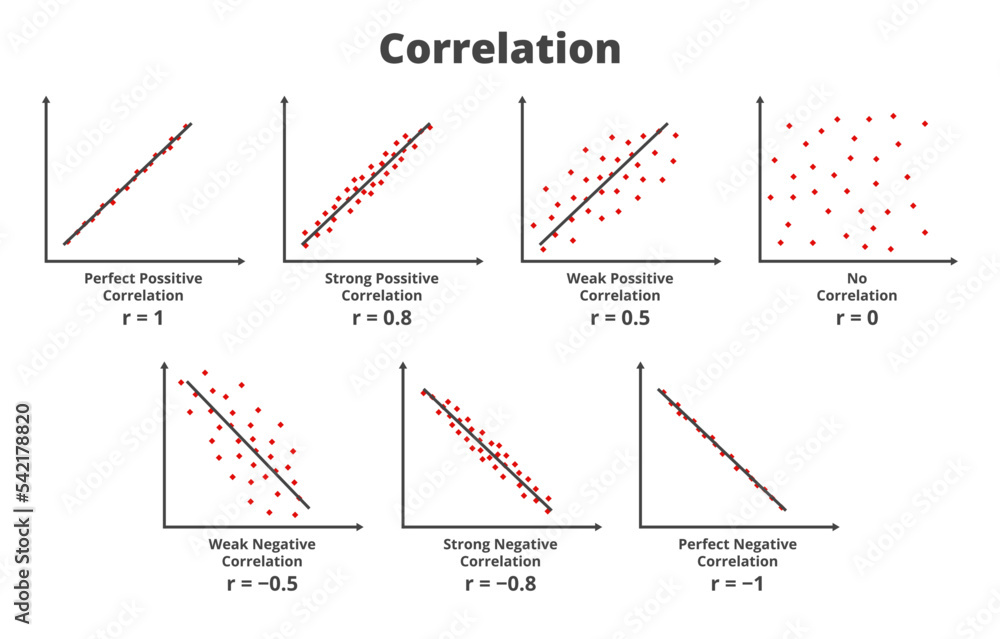
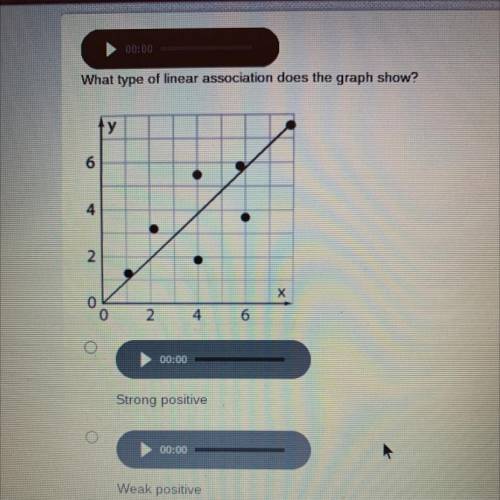
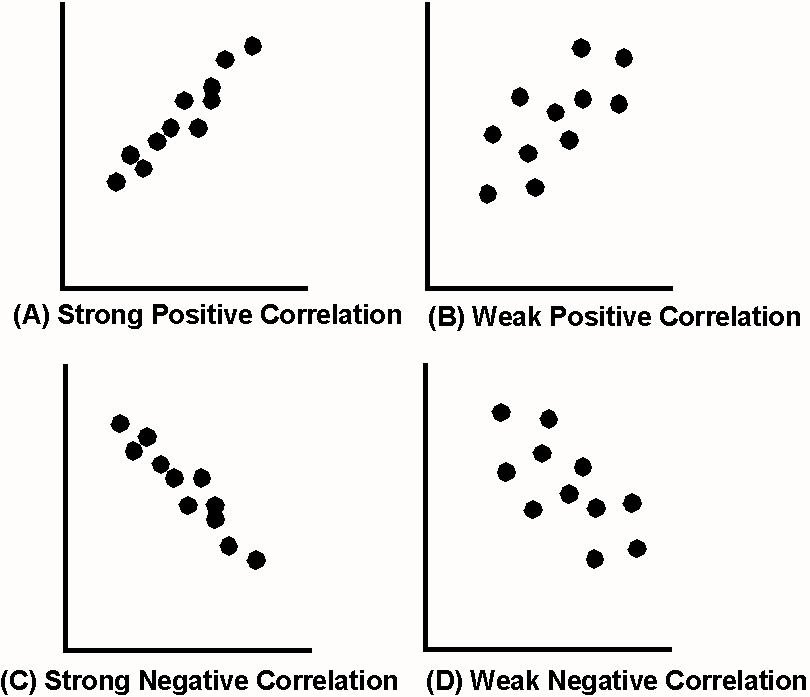
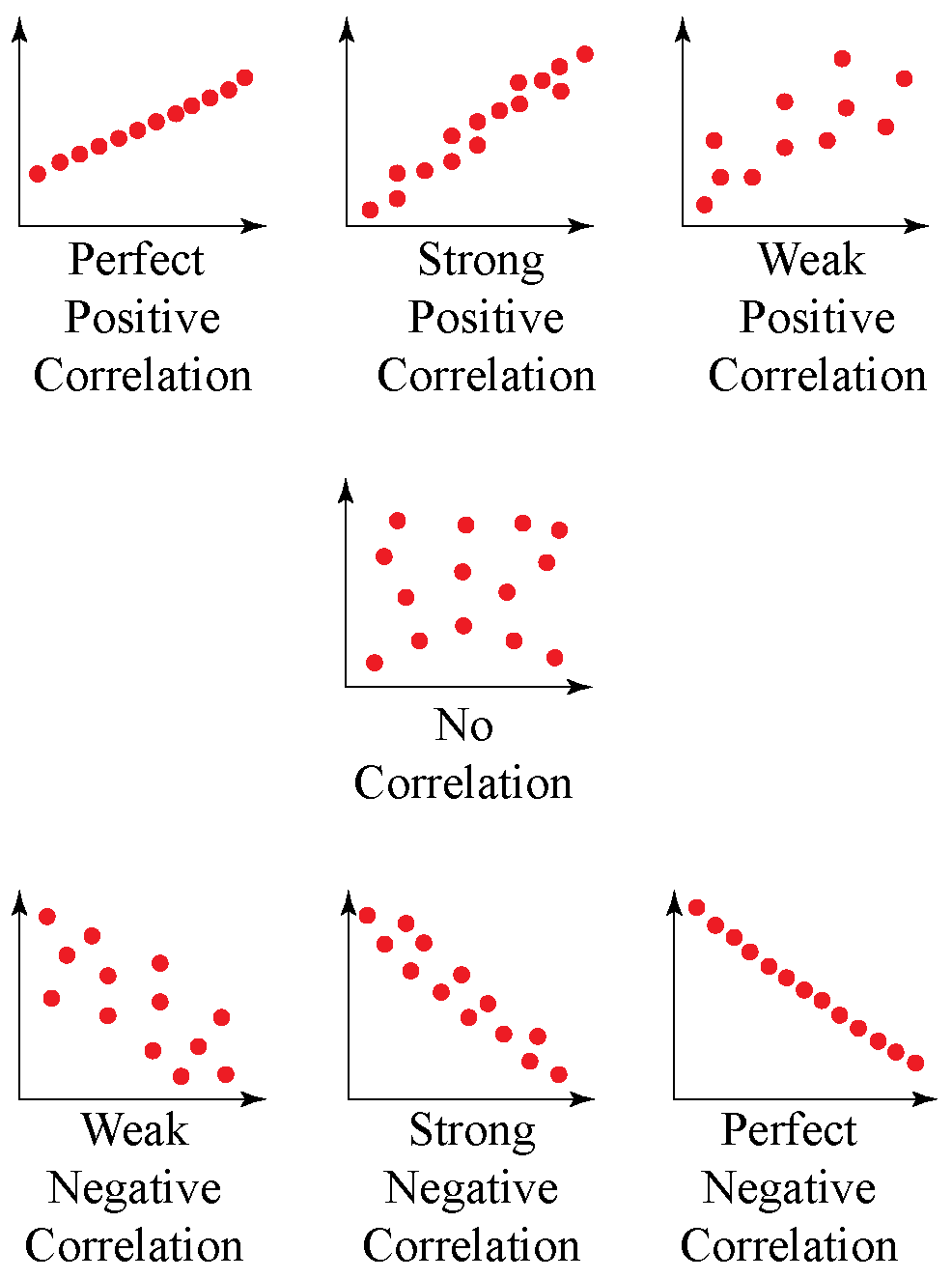
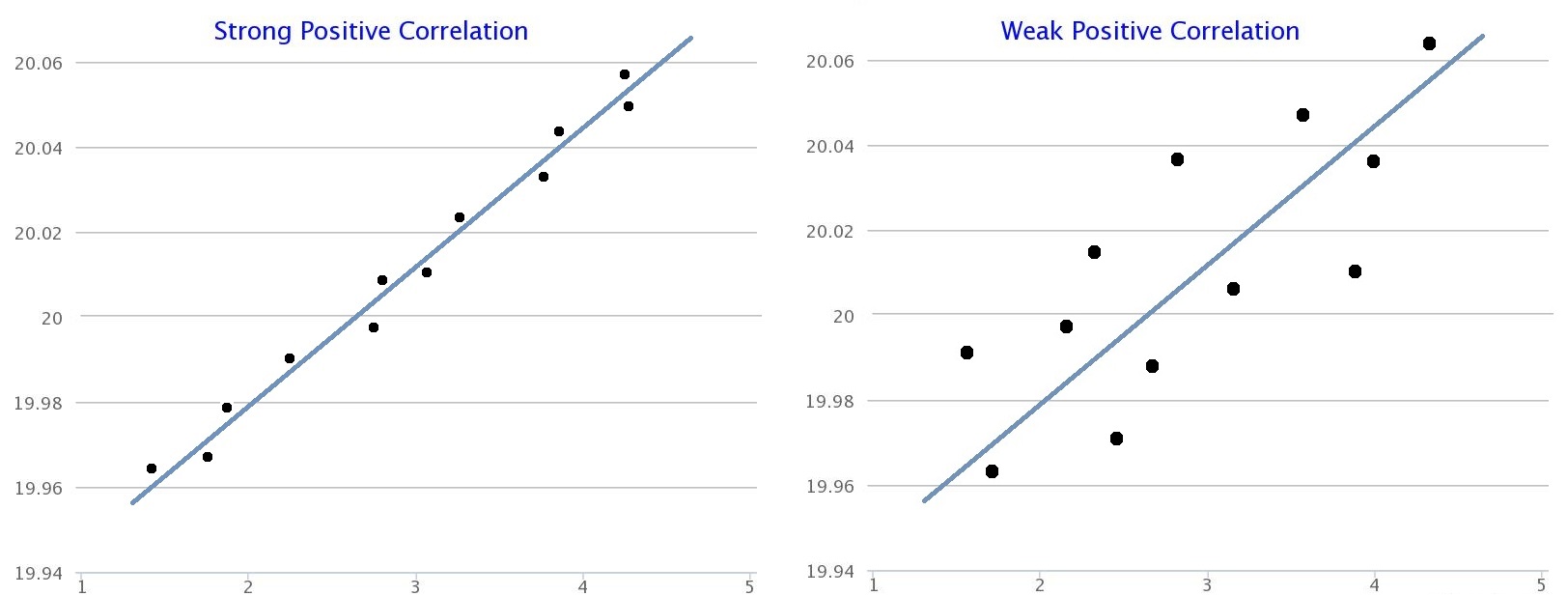
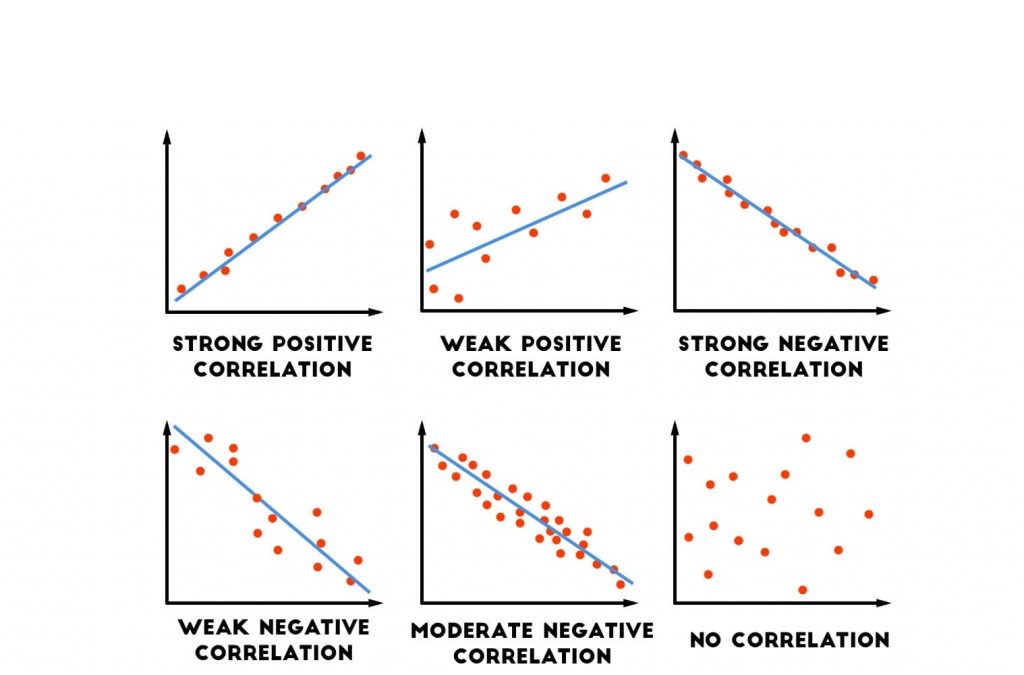
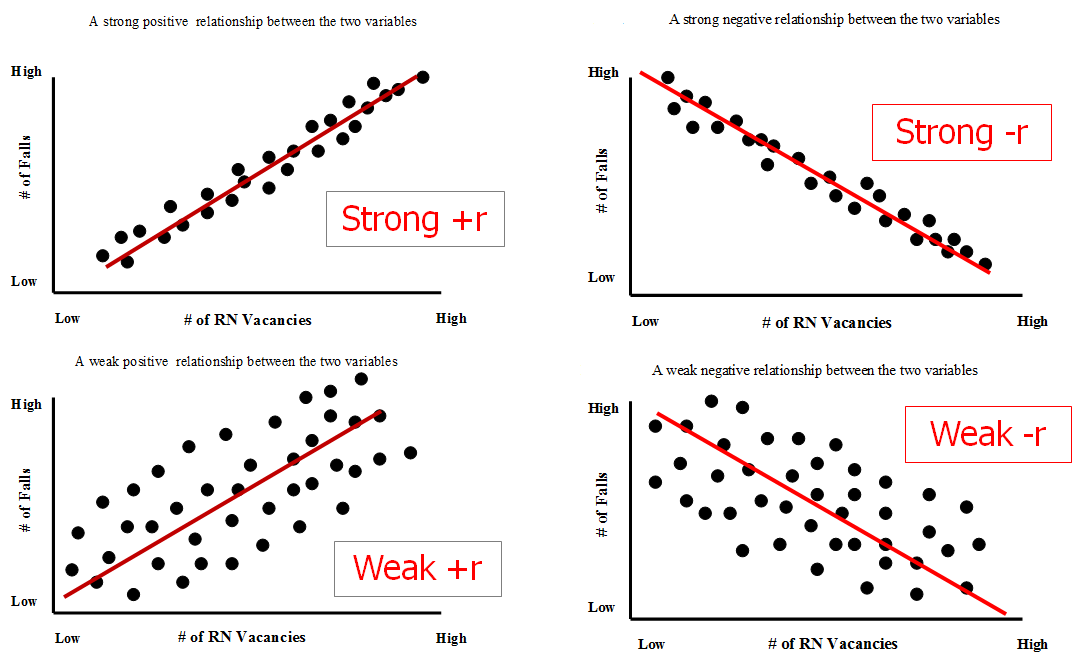
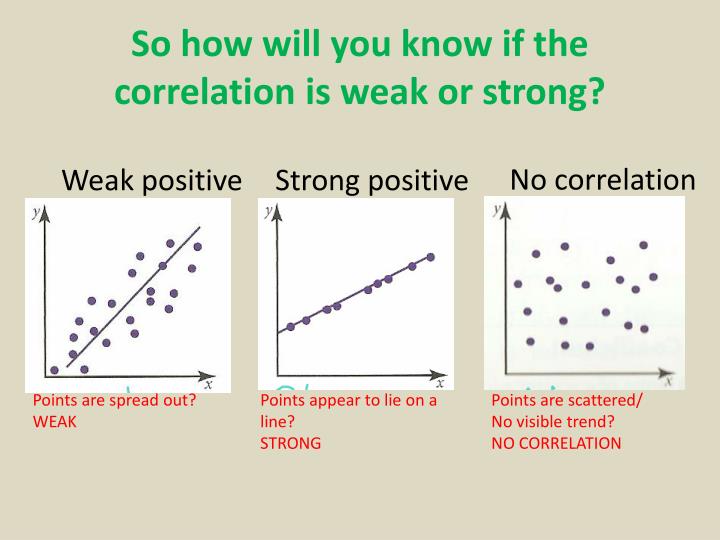
How do you know if a graph is weak or strong. Yes, a graph can, according to the provided definitions, definitely be both weakly and strongly connected at the same time. Does the association appear to be strong, moderately strong, or weak? How do we determine if a decay is due to weak or strong interaction.
Weak acids have strong conjugate bases, while weak bases have strong conjugate acids. The reaction of the weak acid, acetic. On the following diagram how do we decide that the.
It's also important to include the context of the two variables in the description of these. What is the ph before any acid is. Is it true that as long as any one of the 6 flavour numbers are not conserved, then it must be.
Classify the basicity of potassium hydroxide, koh , based on its reactivity in aqueous solution. Rule of thumb is: The correlation between two variables is considered to be weak if the absolute value of r is between 0.25 and 0.5.
Scatterplots are really good for helping us see if. Do there appear to be any data points that are unusually far away from the general pattern? 5 november 2017 by tejvan pettinger.
However, the definition of a “weak”. What is the best description of this relationship? You're simply applying the definition:
Cross elasticity of demand (xed) measures the percentage change in. B) the equivalence point of a titration of a strong acid with a strong base may be observed with an indicator whereas the equivalence point of a titration of a weak. There are 3 types of connectivity when talking about a directed graph g g.
Positive and negative associations in scatterplots. Your example is exactly such a graph. In this video, i'll talk about the differences between weak and strong correlation coefficient coefficient.
As shown in the above two reactions, if ha is a weak acid, then its. As well as what zero correlation looks like. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion.
A dashed line means that the relationship is strong, whereas a solid line means that the relationship is weak.